Partial 1 H NMR spectra of a) PO-5 (600 MHz, 298 K, Cl2CDCDCl2); b)... | Download Scientific Diagram
UV/Vis (CH2Cl2, l = 0.1 cm, 298 K) spectra overlay of a solution of 5... | Download Scientific Diagram

An ideal monoatomic gas (Cv = 1.5 R) initially at 298 K and 1.013 atm expands adiabatically irreversibly until it is in equilibrium with a constant external pressure of 0.1013 atm. The
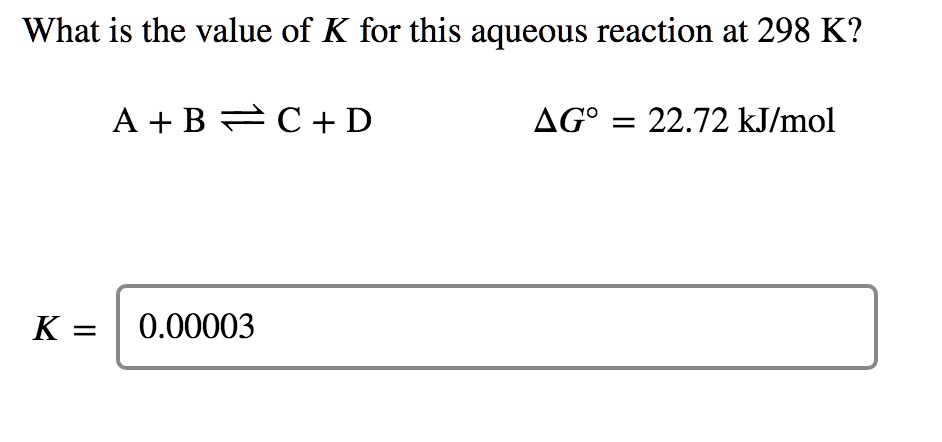
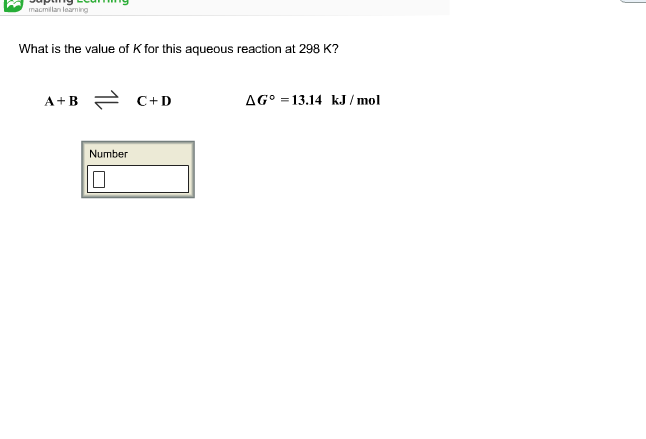
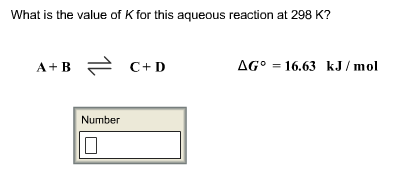
SOLVED: What is the value of K for this aqueous reaction at 298 K? A + B = C + D ΔGo = 22.72 kJ/mol K = 0.00003

convert the following temperature into Celsius scale 298 Kelvin 300 Kelvin 98 degree fahrenheit - Brainly.in

Pls solve this: (ans - c) Q If at 298 K the bond energies of C H, - Chemistry - Chemical Kinetics - 12551821 | Meritnation.com

1 H NMR spectra (500 MHz, 298 K) of (a) L1, (c) L2 in CDCl 3 and (b)... | Download Scientific Diagram
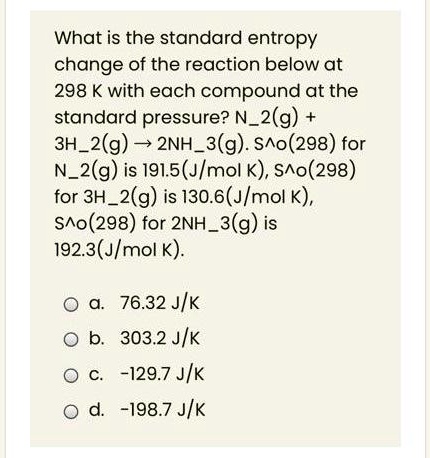
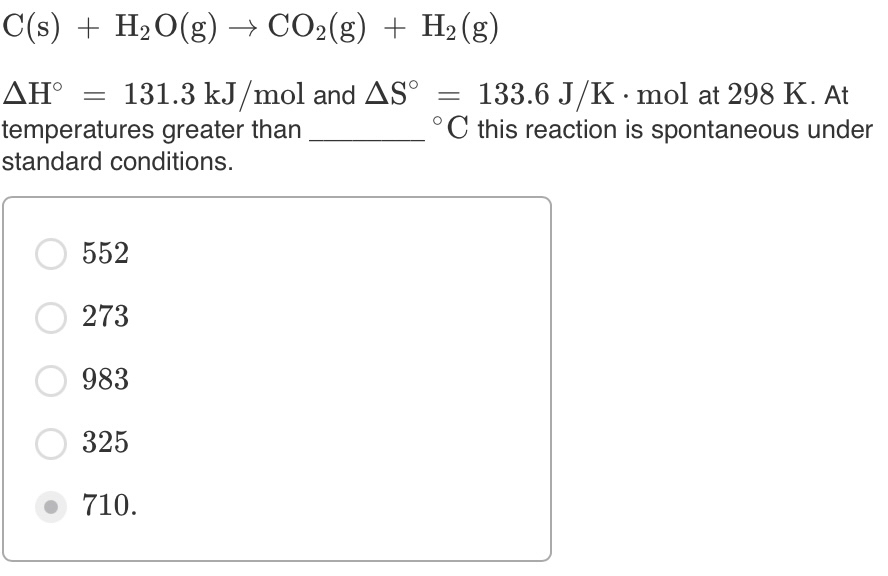
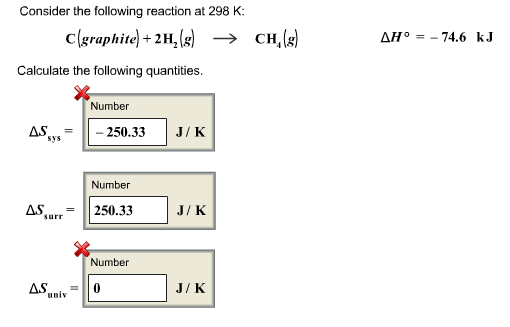
SOLVED: What is the standard entropy change of the reaction below at 298 K with each compound at the standard pressure? Nâ‚‚(g) + 3Hâ‚‚(g) â†' 2NH₃(g). ΔS°(298) for Nâ‚‚(g) is 191.5 (J/mol·K),
6) G (kJ/mol) values at 298 K for Ag+ (aq), Cl (aq) and AgCI (s) are 77, 129 and 109 respectively for the given reaction, Ag (aq) + C (aq) AgCI(s) Then
K, for A(g)+ B(g) 3C(g) is 30 at 298 K. If a twolitre vessel contains 1, 2 and 5 moles of A, B and Crespectively, the reaction at 298 K shall