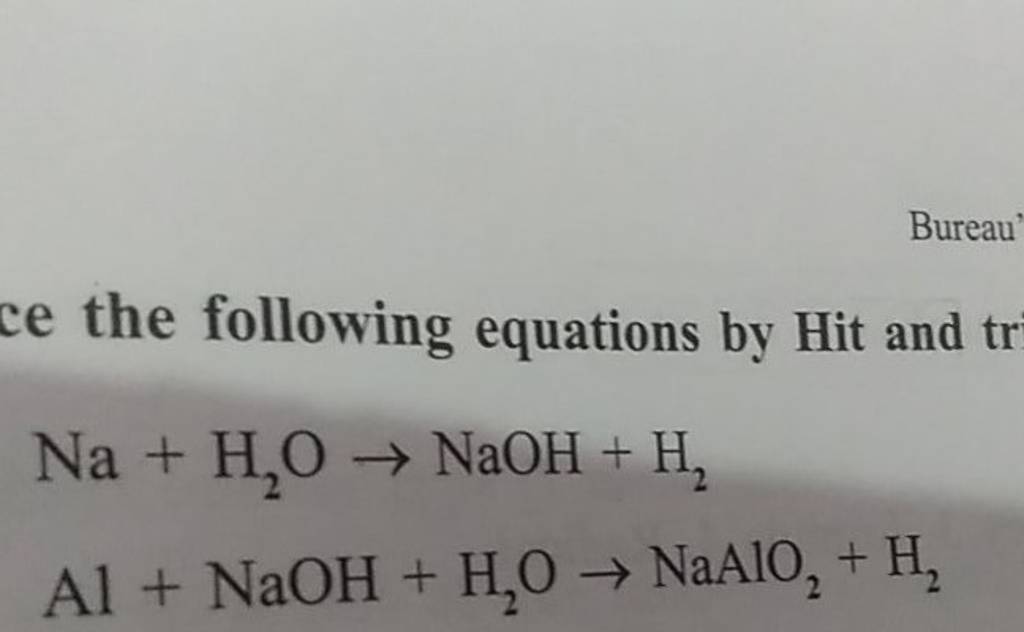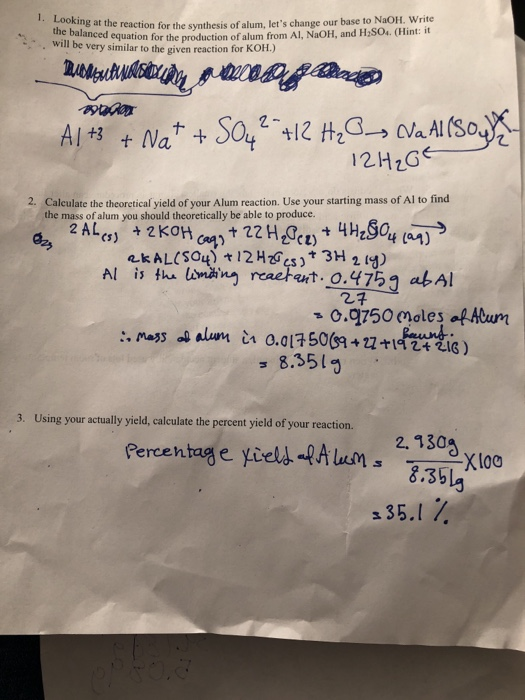Li , K , Ca , Na , Mg , Al , Zn , Fe , Pb , Cu , Hg , Ag I . The reaction Zn(s) + Cu(NO3)2(aq)→ Cu(s) +

XRD analysis data for initial Al and synthesized Cо(Al), Cо(Al)NaOH,... | Download Scientific Diagram

Ca , Na , Al , Cu , Pt , ile HCl ve NaOH tepkimesi ne oluır sırasıyla tepkimesi ne olur hepsi sırasıyla - Eodev.com
![H2O + NaOH + Al = Na[Al(OH)4] + H2 How to balance this redox reaction by oxidation number method ? - Brainly.in H2O + NaOH + Al = Na[Al(OH)4] + H2 How to balance this redox reaction by oxidation number method ? - Brainly.in](https://hi-static.z-dn.net/files/d70/9f97b4b09b95702171bcd0b21f098cc0.jpg)
H2O + NaOH + Al = Na[Al(OH)4] + H2 How to balance this redox reaction by oxidation number method ? - Brainly.in

Al + NaOH gives Na Al O2 + H2 how to balance this equation - Science - Materials Metals and Non-Metals - 14177733 | Meritnation.com
Which of the following statements is incorrect?AIPMT (Mains) 20111(1) Aluminium reacts with excess NaOH to giveAl(OH)_3,(2) NaHCO_3, on heating gives Na_2CO_3 (3) Pure sodium metal dissolves in liquidammonia to give blue solution(4)

NaOH+Al=Al(OH)3+ Na Balanced Reaction||Sodium hydroxide+Aluminium=Aluminium hydroxide+sodium Balance - YouTube

![Balance : NaOH + Al + H(2) O to Na[Al (OH)(4) ] + H(2) Balance : NaOH + Al + H(2) O to Na[Al (OH)(4) ] + H(2)](https://d10lpgp6xz60nq.cloudfront.net/ss/web-overlay-thumb/13042953.webp)
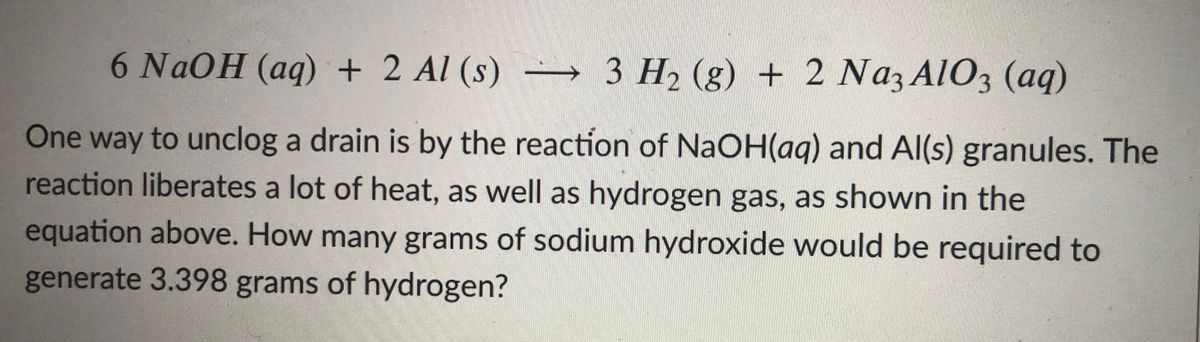




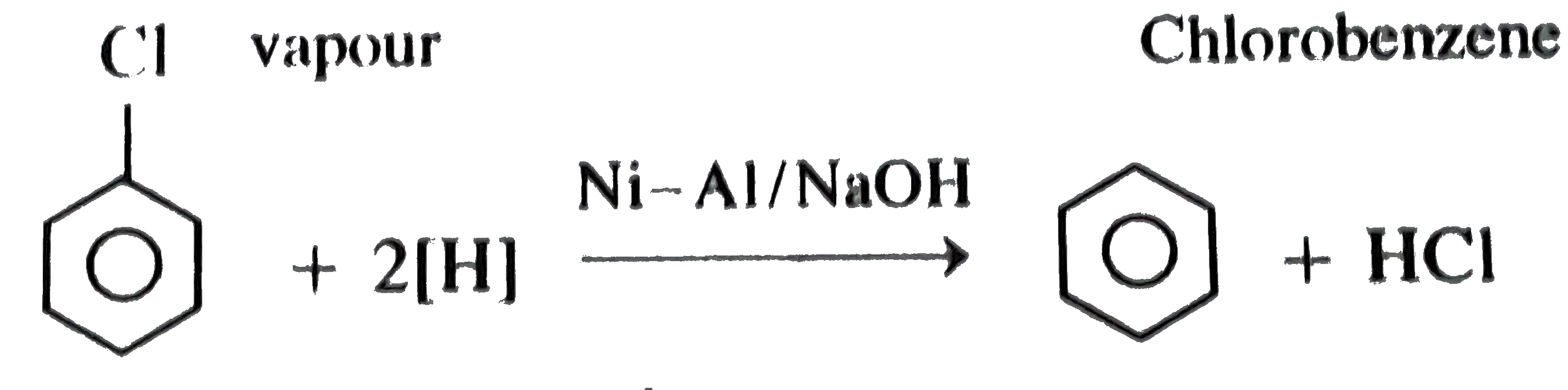


![TÌM HIỂU] Phương Trình Al NaOH H2O TÌM HIỂU] Phương Trình Al NaOH H2O](https://truongkinhdoanhcongnghe.edu.vn/wp-content/uploads/2023/07/al-naoh-h2o-1-min.jpg)