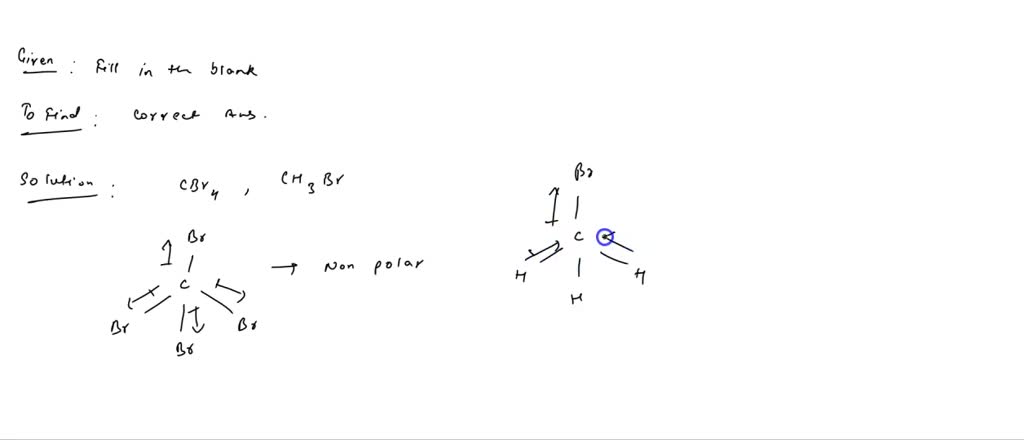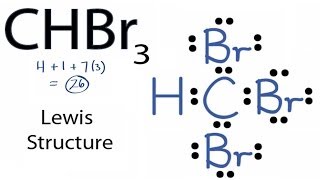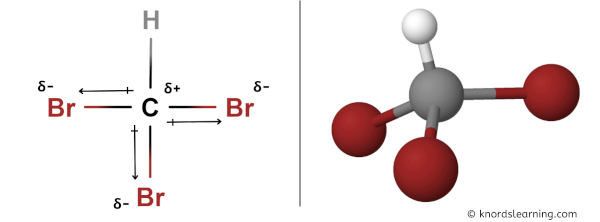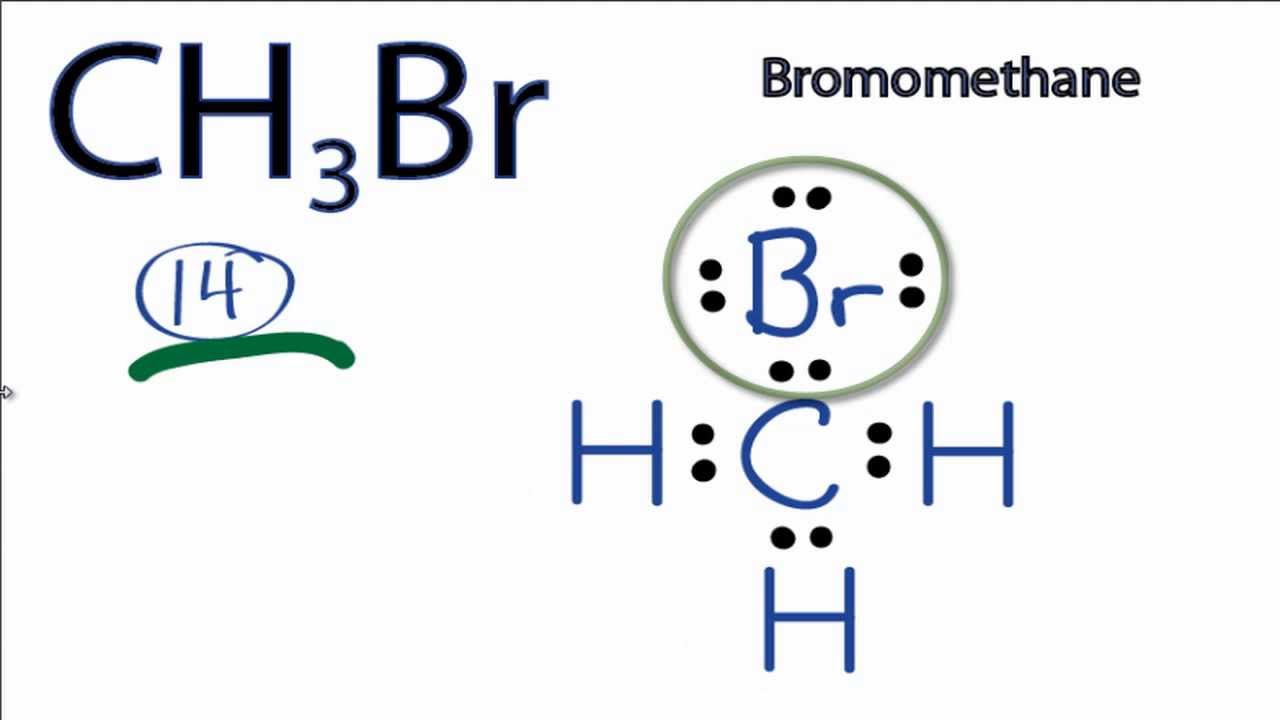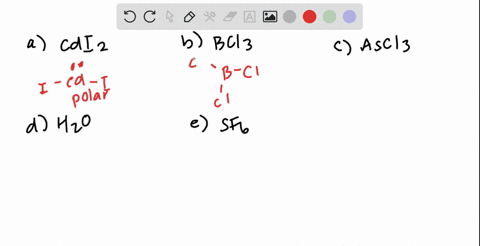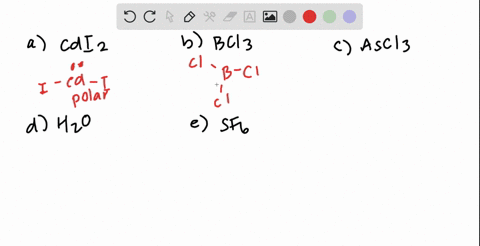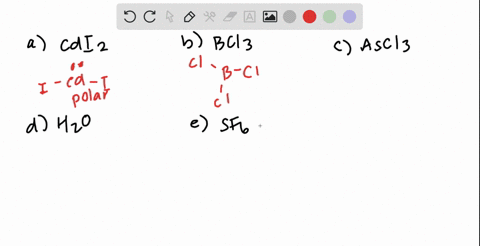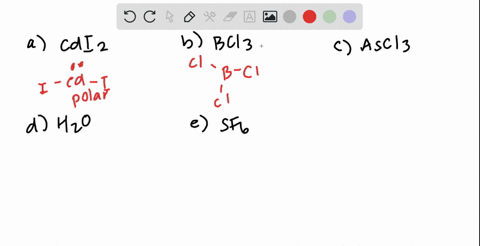
SOLVED: Which of the following molecules are polar? Why? (a) CH4 ; (b) CH3 Br ; (c) CH2 Br2 ; (d) CHBr3 ; (e) CBr4. | Numerade
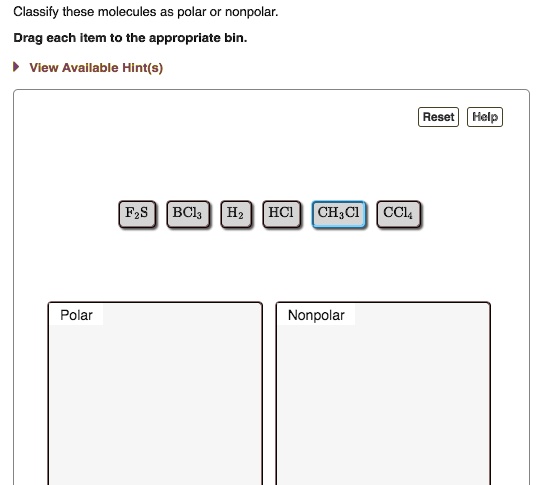
SOLVED: Classify these molecules as polar or nonpolar: Drag each item to the appropriate bin: View Available Hint(s) Reset Help F2S BCl3 HCl CH2Cl CCl4 Polar Nonpolar
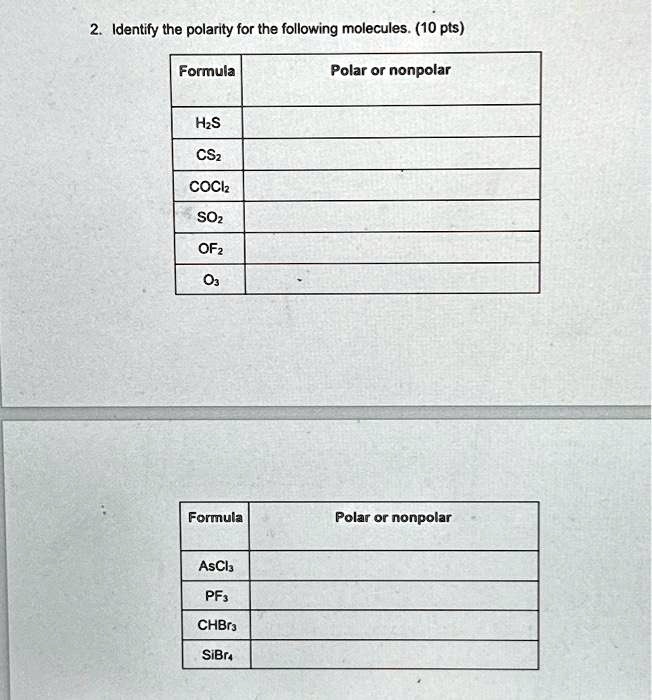
SOLVED: Texts: 2. Identify the polarity for the following molecules. 10 pts Formula Polar or nonpolar H2S CS2 COCl2 SO2 OF2 O3 Formula Polar or nonpolar AsCl3 PF5 CHBr3 SiBr4

Bond and Lone Pairs Valence electrons are distributed as shared or BOND PAIRS and unshared or LONE PAIRS. • •• H Cl shared or bond pair lone pair (LP) - ppt download

Which of the following statements can be used to prove that carbon is tetrahedral? a.) CH3Br does not have constitutional isomers b.) CBr4 does not have a dipole moment c.) CH2Br2 does

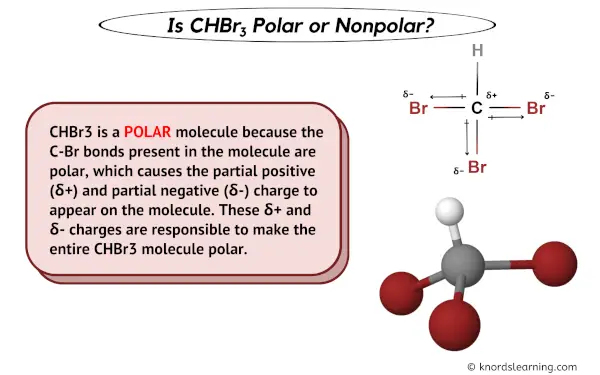
SOLVED: Text: Indicate whether each of the following would be polar or nonpolar. CHBr3, CO2, CH2ClCH2, BrC=CBr