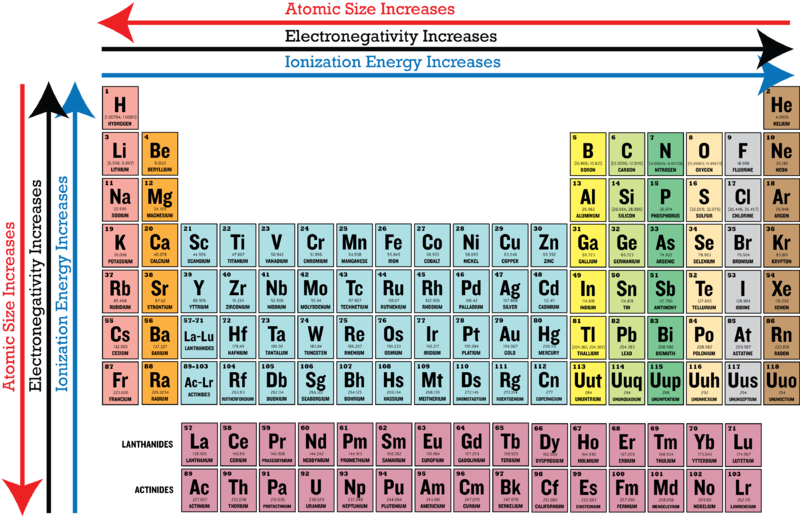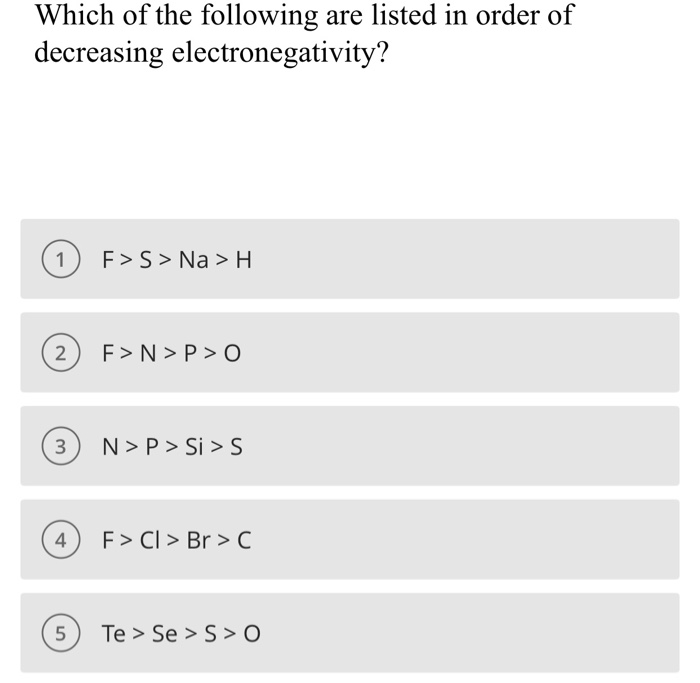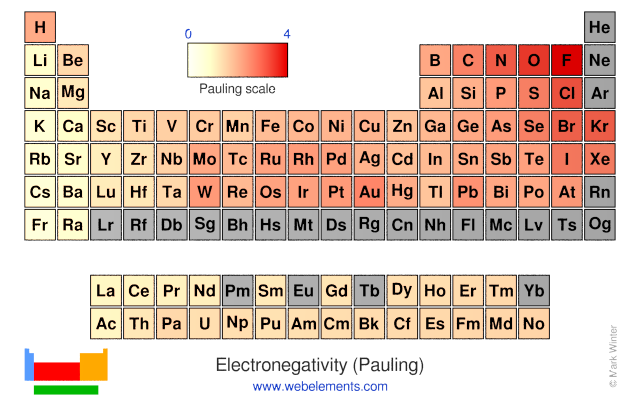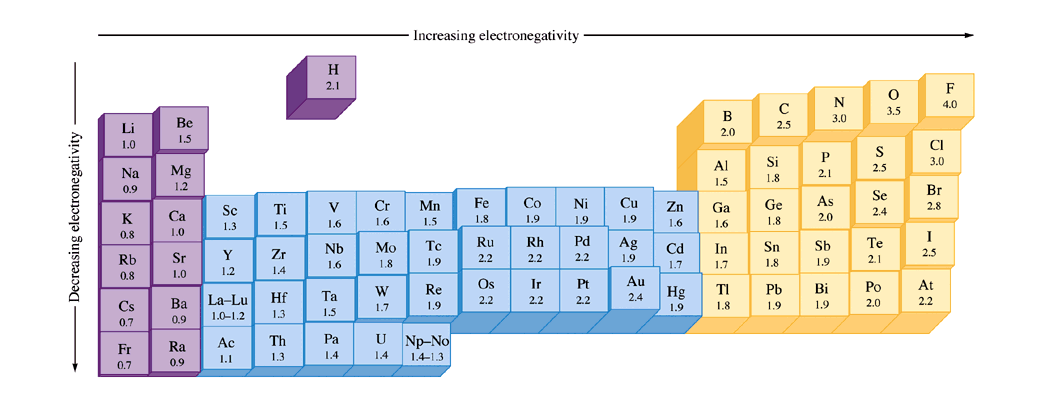
What trend in electronegativity do you see as you go down a group/family on the periodic table? | Socratic

F has the highest electronegativity among the group 17 elements (i.e. ns^(2) np^(2) np^(5) type)... - YouTube

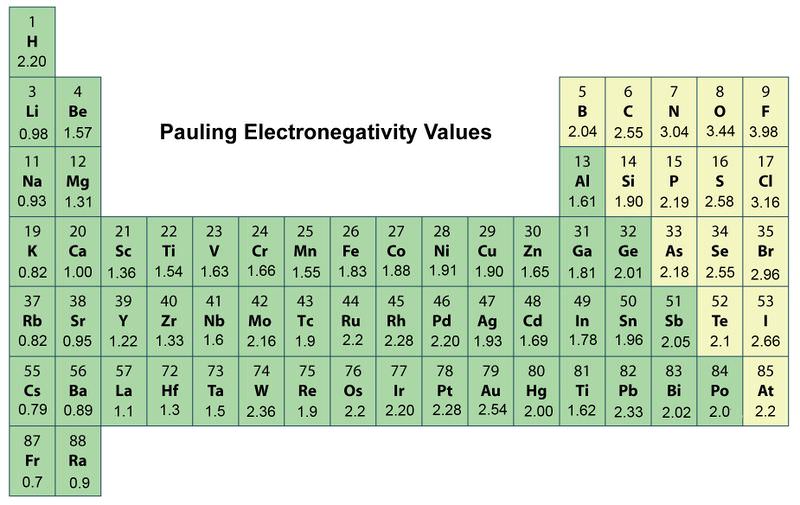
Electronegativity of `F` on Pauling scale si `4.0` Calculate its value on Mulliken scale . - YouTube
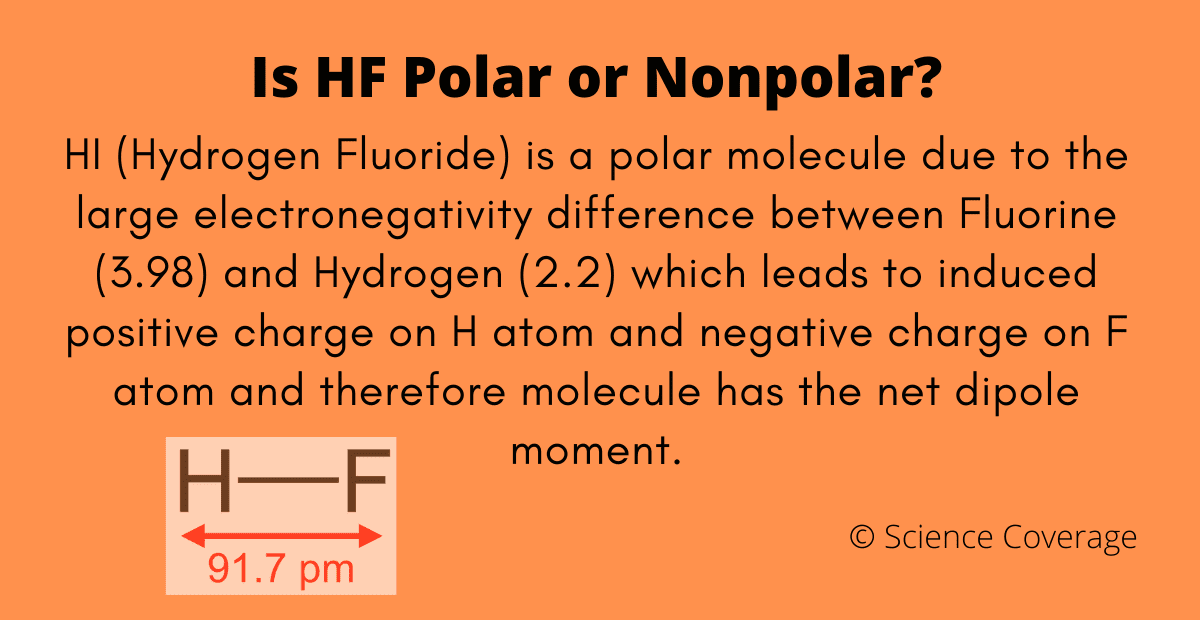
Welcome to Chem Zipper.com......: The electronegativity of F and H are 4.0 and 2.1 respectively. The percentage ionic character in H and F bond is.

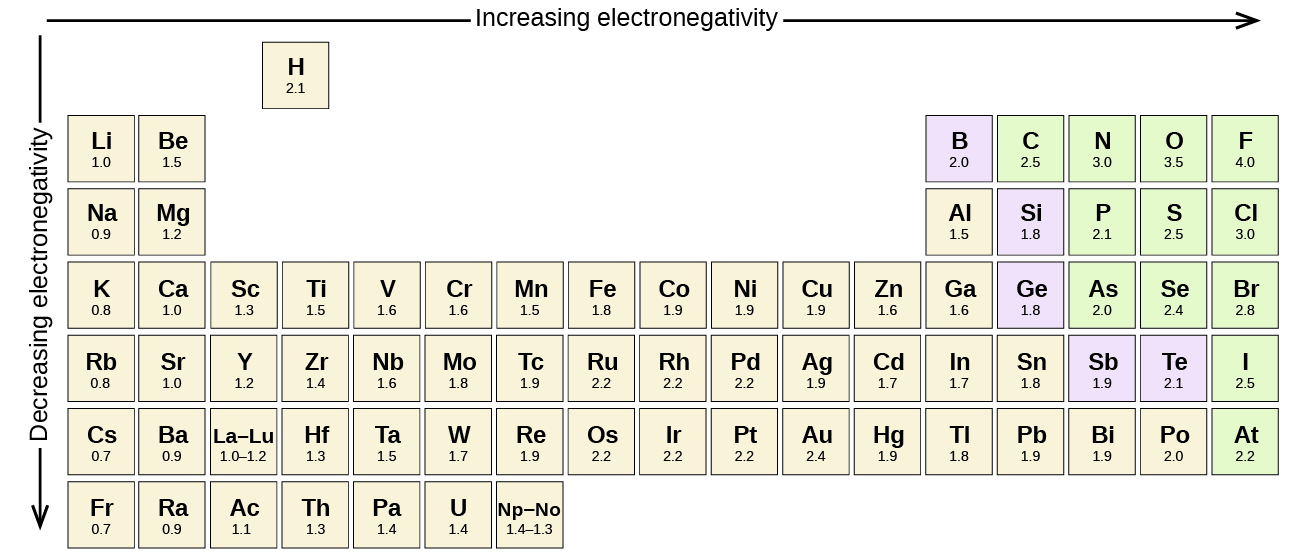
SOLVED: For the elements Cs, F, and Cl, the order of increasing electronegativity is: a) F < Cl < Cs b) Cs < Cl < F c) Cl < Cs < F

Using periodic trends, arrange the following atoms in order of increasing electronegativity: N, Br, Cl, O and F. a) Br < O < N = Cl < F b) Br < N =

Electronegativity Enhanced Strong Metal–Support Interaction in Ru@F–Ni3N for Enhanced Alkaline Hydrogen Evolution | ACS Applied Materials & Interfaces